Background:When an uninsured, undocumented immigrant presents with an emergency medical condition, they may qualify for Emergency Medicaid. Notably, the care and services related to HSCT are not covered, except in California and Washington state. Therefore, many patients with MRD-positive or relapsed ALL who may otherwise be good candidates for HSCT are unable to receive it. This may lead to months of resource-intensive treatment without the chance of cure. Here we present 3 such patients and their course of treatments and outcomes.
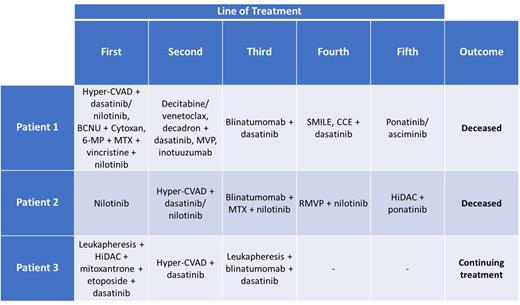
Patient 1:A28-year-old male from Mexico was diagnosed with Ph+ B-ALL and treated with three cycles of hyper-CVAD + dasatinib. BCR-ABL PCR remained elevated at 27.9% and the patient was switched to nilotinib for 5 more cycles of hyper-CVAD; nilotinib was continued until later relapse. He began maintenance BCNU/cytoxan, then several cycles 6-MP/MTX/vincristine. On day +554 after diagnosis, he relapsed and started decitabine/venetoclax, then decadron + dasatinib, then MVP, followed by 3 cycles of inotuzumab. Later, he started blinatumomab on day +648, which was discontinued after 22 days due to increasing peripheral blasts. He then started SMILE, then CCE. As his condition worsened he was put on ponatinib, then asciminib before expiring on day +940.
Patient 2: A37-year-old male from Mexico was diagnosed with CML and started on nilotinib. On day +212 he progressed to lymphoid blast phase CML with CNS involvement and received hyper-CVAD/dasatinib; was switched to nilotinib on cycle 4 due to concerns about pleural effusion while hospitalized for PNA. On day +447 he started blinatumomab + IT MTX + nilotinib. A brain MRI on day +544 suggested carcinomatous meningitis and he got 4 cycles RMVP. He then switched to ponatinib and HiDAC for 8 cycles despite continued ALL in CSF. He was admitted for worsening ALL-induced headache on day +1016 and imaging discovered multifocal pneumonia. He then had seizure-like activity consistent with metabolic encephalopathy and expired on day +1055.
Patient 3: A28-year-old male from Ecuador was diagnosed with Ph+ mixed phenotype (B/myeloid) acute leukemia. The patient underwent leukapheresis and started HiDAC + mitoxantrone + etoposide + dasatinib. Subsequent bone marrow biopsy showed 1% blasts, with BCR-ABL PCR at 1.4%. He was admitted on day +170 with Ph+ B-ALL relapse and underwent leukapheresis + 2 cycles hyper-CVAD. On day +255 he began blinatumomab for 3 cycles. He continues to receive treatment for his B-ALL at this time.
See table 1 below.
Discussion: There is an increasing population of undocumented immigrants entering the United States and receiving treatment for ALL while on emergency Medicaid. It is important to understand the special considerations in this population, including the inability to have HSCT authorized as a treatment option. Studies comparing the costs of HSCT to chemotherapy have found that chemotherapy is cheaper at the expense of patient mortality. Therefore, HSCT's incremental cost-effectiveness ratio, measured as cost per quality-adjusted life years, may still be favorable. As our case series demonstrates, extensive resources including traditional chemotherapies, immunotherapies like blinatumumab, and targeted oral drugs, can further increase the costs relative to HSCT. Moreover, the National Bureau of Economic research has calculated that undocumented workers add about $45,000 per worker to the US GDP annually. Therefore, offering HSCT and allowing them to continue to work may be a reasonable financial option. Likewise, many undocumented adults are the parents of US citizen minors who would benefit economically from their survival. For consideration we will explore cost analysis to further elucidate the potential benefit of allowing coverage of HSCT.
Disclosures
Liu:Beigene: Speakers Bureau; Rigel: Speakers Bureau. Seiter:Servier: Speakers Bureau; Alexion: Honoraria; Incyte: Speakers Bureau; CTI: Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Rigel: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Blueprint: Speakers Bureau. Steinberg:MorphoSys: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal